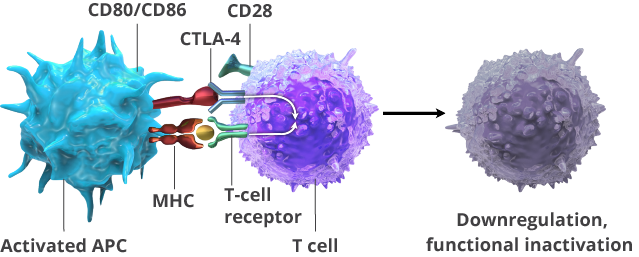
CTLA-4 is an immune checkpoint that is expressed early in the T-cell activation process. It competes with the CD28 receptor, which causes activation of T cells, dampening that activation. Tumor cells can express the CD80/CD86 ligands that bind with CTLA-4, inhibiting the activation of T cells.
Ipilimumab (Yervoy®)
Ipilimumab is a monoclonal antibody that binds to CTLA-4, blocking its interaction with its CD80/CD86 ligands. This augments T-cell activation and proliferation, including the activation and proliferation of tumor-infiltrating T-effector cells. In addition, the inhibition of CTLA-4 signaling can also reduce T-regulatory cell function, which may contribute to a general increase in T-cell responsiveness, including the anti-tumor immune response.
Ipilimumab has a variety of indications for the treatment of various cancers, including melanoma and renal cell carcinoma. It is indicated for the treatment of adult and pediatric patients 12 years of age and older with microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) metastatic colorectal cancer that has progressed following treatment with a fluoropyrimidine, oxaliplatin, and irinotecan, in combination with nivolumab.
The prescribing information for ipilimumab has a boxed warning related to the potential for severe immune-mediated adverse reactions, including enterocolitis, hepatitis, dermatitis, neuropathy, and endocrinopathy. When used as a single agent, the most common adverse reactions occurring in 20% or more of the patients in clinical trials were fatigue, diarrhea, pruritus, rash, and colitis.
Checkpoint Inhibition with CTLA-4