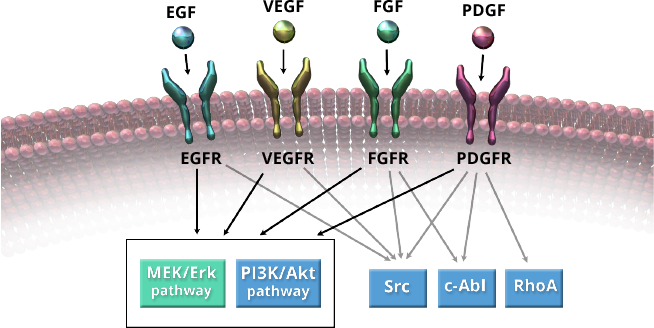
Activation of multiple signaling pathways in the tumor microenvironment contribute to tumor growth and angiogenesis, including pathways activated by tyrosine kinase receptors such as vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR). Although a variety of kinase inhibitors have been developed to inhibit VEGFR, many other growth factors and receptors have been shown to work in a complementary and coordinated fashion to regulate tumor growth and angiogenesis.
Regorafenib is a small molecule inhibitor of multiple kinases including VEGFR, FGFR, PDGFR, BRAF, KIT, and RET. These kinases are involved with various processes that support tumor growth, angiogenesis, metastasis, and tumor immunity.
Regorafenib has indications in patients with mCRC, gastrointestinal stromal tumors, and hepatocellular carcinoma. It is indicated for the treatment of patients with mCRC who have been previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy; an anti-VEGF therapy; and if RAS wild-type, an anti-EGFR therapy.
The regorafenib prescribing information has a boxed warning related to the potential for hepatotoxicity. Severe and sometimes fatal hepatotoxicity has been observed in clinical trials. Physicians should monitor hepatic function prior to and during treatment. In addition, they should interrupt then reduce or discontinue regorafenib therapy if hepatotoxicity occurs. In patients receiving regorafenib, hepatotoxicity may be manifested as elevated liver function tests or hepatocellular tissue destruction, depending upon the severity and persistence of the reaction.